ABCSG 65 / DEFINTIVE Study Details
Diagnostic HER2DX-guided treatment For patIeNts wIth early-stage HER2-posiTIVE breast cancer
An international, multicenter, prospective, two-arm, randomized, open-label Phase III study designed to demonstrate that personalized treatment decisions in HER2-positive early-stage breast cancer using the HER2DX® diagnostic test improve quality of life without compromising outcomes and survival rates.
| Study Start: | (global): 11/2024, FPI 11/2024 (national): 07/2025 |
| Coordinating Investigator AT: | Rupert Bartsch, Vienna |
| Participants: | 315 (global), 70 (national) |
| Study Design: (Click to enlarge) |
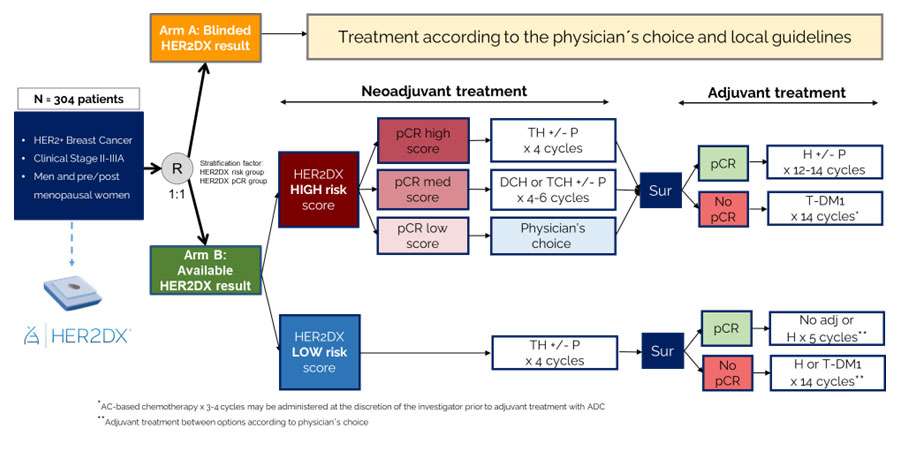 |
Treatment
Patients who meet randomization eligibility criteria will be randomized in a 1:1 ratio:
- Arm A: Blinded HER2DX result – Treatment according to the physician’s choice and local guidelines
- Arm B: Available HER2DX result – HER2DX guided treatment
HER2DX test results will be shown only for patients randomized to ARM B.
Randomization will be stratified by the following factors:
- HER2DX risk group (high vs. low groups): Risk of recurrence following curative intent therapy.
- HER2DX pCR likelihood group (high vs. medium vs. low groups): The likelihood of the tumor completely disappearing after undergoing neoadjuvant trastuzumab-based CT.
Primary Objectives
- To determine whether there is an improvement in the health-related quality of life (HRQoL) following tailored treatment by HER2DX compared with standard of care treatment.
- To evaluate whether the strategy of tailoring treatment by HER2DX presents a similar rate of responses than the standard of care treatment.
Secondary Objectives
- To evaluate other HRQoL patient-reported outcomes (functional and symptom values) following tailored treatment by HER2DX compared with standard of care treatment.
- To evaluate whether strategy of tailoring treatment by HER2DX presents similar effectiveness outcomes than the standard of care treatment.
- To evaluate the association between HER2DX scores and efficacy outcomes
- To assess the safety and tolerability of test guided treatment and their corresponding standard treatment.
- To evaluate patient experience in control arm compared to those treated using the test and assess whether the inclusion of HER2DX test has an impact on patient experience.
- To analyze the economic impact of the HER2DX test.
- To evaluate whether the potential treatment de-escalation following tailored treatment by HER2DX could have impact in the work productivity.
Exploratory Objective
- To explore biomarkers identified at baseline using available clinical or pathological data, or remaining RNA from tumor samples.
Patient Population
- The target population of interest in this study consists of patients with HER2- early breast cancer stage II-IIIa.
- This trial will be conducted in men and pre/postmenopausal women.
- Patients have not received any prior treatment for breast disease.
- Patients enrolled in this protocol are permitted to participate in additional parallel investigational drug(s) and/or device(s) studies while on treatment as long as it does not interfere with the treatment, tests and follow-up of the DEFINITIVE protocol.
Share on

